CardioComm Solutions, Inc.
CardioComm Solutions, Inc.
CardioComm Solutions, Inc. has released a wireless (BLE) handheld ECG monitor that pairs with the mSafety watch. The device is the HeartCheck™ CardiBeat which is cleared in Canada, the US and the EU as a Class II medical device that can be sold under physician direction or directly to consumers. The device can record a Lead I or Lead II ECG with a standard recording duration of 30 seconds.
Commercially, the HeartCheck™ CardiBeat is used with its free mobile application GEMS™ Mobile. The GEMS™ Mobile software has been embedded into the mSafety watch eliminating the need for the use of a smartphone. The total solution enables a user to activate the technology in the mSafety watch, record a 30 second ECG while holding the HeartCheck™ CardiBeat and to view the ECG on the mSafety watch during the recording.
Typical use cases include:
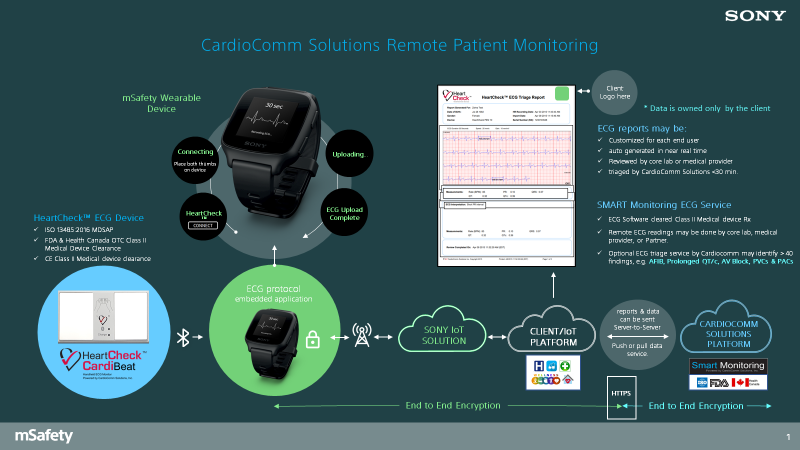
Download an overview of how CardioComm uses Sony's mSafety.
Copyright 2025 Sony Network Communications Europe.